“Available” calcium, soil pH, and fearmongering
I did an experiment in a greenhouse in which I grew creeping bentgrass in four different sands. I collected all the clippings and measured what was in them. And I tested the pH of the sands, and I did soil tests to measure the soil nutrient content.

There is almost always enough calcium in the soil, even in sand rootzones with low CEC, to provide all the calcium the grass requires.
Tom Margetts asked a good question about “available” calcium, especially in calcareous soils. Calcareous soils have free calcium carbonate. That is, they have some amount of calcium carbonate in the solid phase. And one sometimes hears that the calcium in calcareous soils is abundant, but isn’t available. That is a complete misunderstanding of the situation.
Obviously the grass roots don’t take up calcium (or any element) from the solid phase. The roots take up elements that are in soil solution. The idea that calcium in calcareous soils is somehow unavailable to grass is ridiculous fearmongering.
The reason there is solid phase calcium in calcareous soil is because the soil solution is saturated with calcium. In fact, it is often super-saturated, having more calcium in solution than would be expected by thermodynamic calculations. Simply put, there is plenty of calcium in the soil solution of calcareous soils.
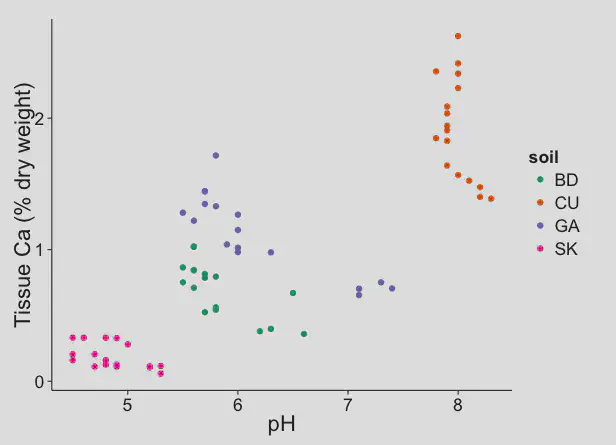
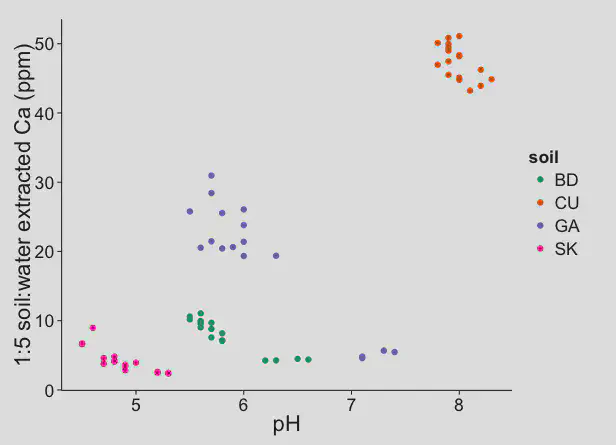
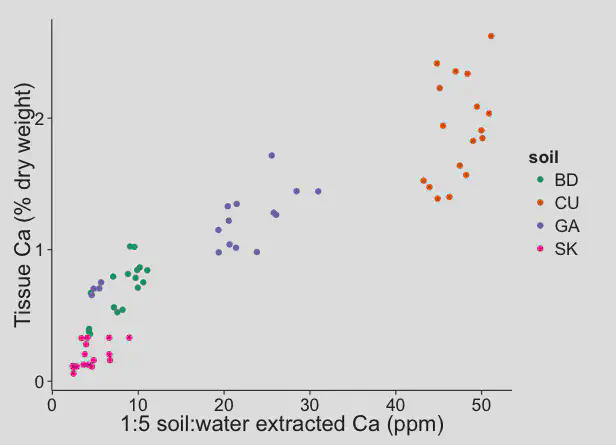
Data from the experiment with the four different sands shows what we can expect in turfgrass soils. These next three charts show that the calcium availability was highest in the high pH (calcareous) sand.